Thorium (Th) is a silvery-white colored radioactive metal that has the atomic number 90 in the periodic table. It is an Actinoid Metal with the symbol Th. What is Thorium. Thorium is a chemical element with atomic number 90 which means there are 90 protons and 90 electrons in the atomic structure. The chemical symbol for Thorium is Th. Thorium metal is silvery and tarnishes black when exposed to air, forming the dioxide. Thorium is moderately hard, malleable, and has a high melting point. Man-made thorium isotopes isotopeA form of an element that has the same number of protons but a different number of neutrons in the nucleus, giving it a different atomic mass. For example, uranium has thirty-seven different isotopes, including uranium-235 and uranium-238. Are rare, and almost never enter the environment.
Which must occur for the atom of an element to be transformed into an atom of an element whose atomic number is less by two? D.The atom must lose two protons In nature, thorium (atomic number, 90) transforms spontaneously into radium (atomic number, 88), which spontaneously transforms into radon (atomic number, 86). Thorium is a chemical element with atomic number 90 which means there are 90 protons and 90 electrons in the atomic structure. The chemical symbol for Thorium is Th. Atomic Mass of Thorium Atomic mass of Thorium is 232.0381 u.
The Element Thorium
[Click for Isotope Data]

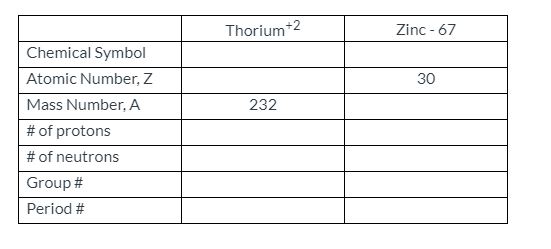
Atomic Number: 90
Atomic Weight: 232.0377
Melting Point: 2023 K (1750°C or 3182°F)
Boiling Point: 5061 K (4788°C or 8650°F)
Density: 11.72 grams per cubic centimeter
Phase at Room Temperature: Solid
Element Classification: Metal
Thorium 232 Atomic Number

Period Number: 7
Group Number: none
Group Name: Actinide
Special Notes: Radioactive
What's in a name? Named for the Scandinavian god of war, Thor.
Say what? Thorium is pronounced as THOR-ee-em or as THO-ree-em.

History and Uses:
Thorium was discovered by Jöns Jacob Berzelius, a Swedish chemist, in 1828. He discovered it in a sample of a mineral that was given to him by the Reverend Has Morten Thrane Esmark, who suspected that it contained an unknown substance. Esmark's mineral is now known as thorite (ThSiO4). Thorium makes up about 0.0007% of the earth's crust and is primarily obtained from thorite, thorianite (ThO2) and monazite ((Ce, La, Th, Nd, Y)PO4).
Thorium is used as an alloying agent to improve magnesium's strength at high temperatures. Thorium is also used to coat tungsten filaments used in electronic devices, such at television sets. When bombarded with neutrons, thorium-232 becomes thorium-233, which eventually decays into uranium-233 through a series of beta decays. Uranium-233 is a fissionable material and can be used as a nuclear fuel.
Thorium oxide (ThO2), one of thorium's compounds, has many uses. It is primarily used in a type of lantern mantel known as a Welsbach mantle. This mantle, which also contains about 1% cerium oxide, glows with a bright white light when it is heated in a gas flame. Thorium oxide has a very high melting point, about 3300°C, and is used to make high temperature crucibles. Thorium oxide is also used to make glass with a high index of refraction that is used to make high quality camera lenses. Thorium oxide is used as a catalyst in the production of sulfuric acid (H2SO4), in the cracking of petroleum products and in the conversion of ammonia (NH3) to nitric acid (HNO3).
Thorium's most stable isotope, thorium-232, has a half-life of about 14,050,000,000 years. It decays into radium-228 through alpha decay or decays through spontaneous fission.
Estimated Crustal Abundance: 9.6 milligrams per kilogram

Estimated Oceanic Abundance: 1×10-6 milligrams per liter
Number of Stable Isotopes: 0 (View all isotope data)
Ionization Energy: 6.08 eV
Oxidation States: +4
Electron Shell Configuration: | 1s2 |
2s2 2p6 | |
3s2 3p6 3d10 | |
4s2 4p6 4d10 4f14 | |
5s2 5p6 5d10 | |
6s2 6p6 6d2 | |
7s2 |
For questions about this page, please contact Steve Gagnon.
Thorium Atomic Number
Thorium, Th, with an atomic number of 90, has an atomic weight of 232.038. Its melting point is 2023 K and its boiling point is in excess of 5000 K. The density of the pure metal form is 11.72 g/cm3. Thorium is a white silvery metal that is stable at surface conditions but over months’ time will oxidize and at the surface will turn gray and subsequently black. The metal is very ductile and can be rolled. Its properties are governed by its purity with the most common contaminant its oxide, which makes the...
